Phase Diagrams
We like think of phase changes as linear transformations. Solid to liquid to gas to plasma, etc. But in reality, that’s not always what happens. Only at standard pressure (1 atm) does water boil at 100 °C and freezes at 0 °C. In fact, the higher up one’s altitude, the lower the atmospheric pressure, which in turn leads to a lower boiling temperature. To boil tea in the Himalayas, one only has to heat the water to around 71 °C. Much of this information about a substance’s freezing and melting points at various pressures can be found on phase diagrams, such as the one below for water.
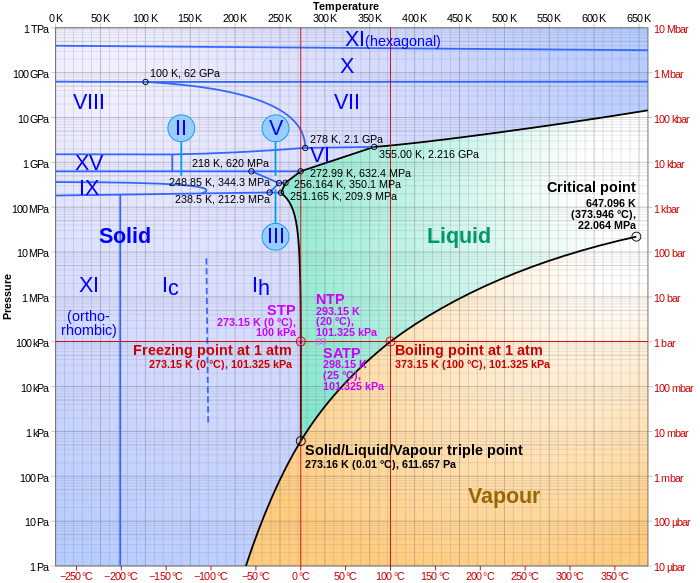
From the diagram, we can see that water turns from a solid to a gas at pressures below about 600 Pa without passing through a liquid phase. Called sublimation, a well known example of it is the carbon dioxide “fog” produced when dry ice is put in warm water. We can also see a triple point, a combination of temperature and pressure (0.01°C and 611.73 Pa) at which ice can be present concurrently in all three states.
Cyclohexane boiling and freezing at the same time:
At even higher pressures, the melting point of water also begins to increase. Water can be solid ice up to temperatures of over 300°C when the pressure is above 10 gigapascals. There are even different types of water ice/vapor that exist at certain pressures.
See also:
